Zeolites
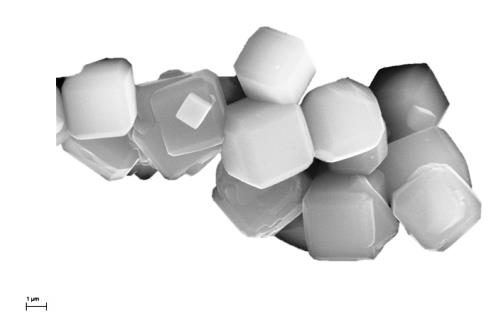
Zeolites are abundant in nature and commonly found in rocks of volcanic origin. When rapidly heated, stones containing Zeolites are observed to release water and appear to boil. The name "zeolite" which is derived from the Greek words Zeo and Lithos reflects this property as it translates as "stone that boils".
Applications

Zeolites have three main properties which are the basis for its many applications:
- Pore structure and chemical composition
- Ion exchange
- Adsorption
Compliance

As zeolites (here defined as cuboidal, crystalline, synthetic and non-fibrous) are being used in many applications of industrial, consumer and even medical nature, it is important to note that they have been extensively tested with regards to their safety aspects form both a toxicological and environmental point of view.



